The Know ALL website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so they may not be exact or complete; Know ALL cannot guarantee the accuracy of translated content. Know ALL and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help. The interviews webpage also uses YouTube, where subtitles and translations are generated automatically by AI; please be advised to exercise caution when precise interpretation is required. For further support with YouTube, visit YouTube Help.
Know ALL webinar | What immunotherapies are available for the treatment of ALL?
Wendy Stock
October 22, 2025
Know ALL hosted a webinar for patients and healthcare professionals (HCPs) on September 22, 2025, titled ‘Immunotherapy for the treatment of ALL: What you need to know’, in which Jaymz Goodman, Charles Mullighan, Wendy Stock, and Jessica Olson discussed immunotherapy for the treatment of acute lymphoblastic leukemia (ALL).
Dr Stock, The University of Chicago, Chicago, US, and Know ALL Ambassador, gave a talk titled ‘What immunotherapies are available for the treatment of ALL?’, in which she provided an overview of approved immunotherapies for the treatment of ALL, current limitations, and future directions.
Quick summary
- Five immunotherapies are currently approved in the US and EU for B-cell ALL (Figure 1).
Figure 1. Immunotherapies currently approved in the US and EU for B-cell ALL
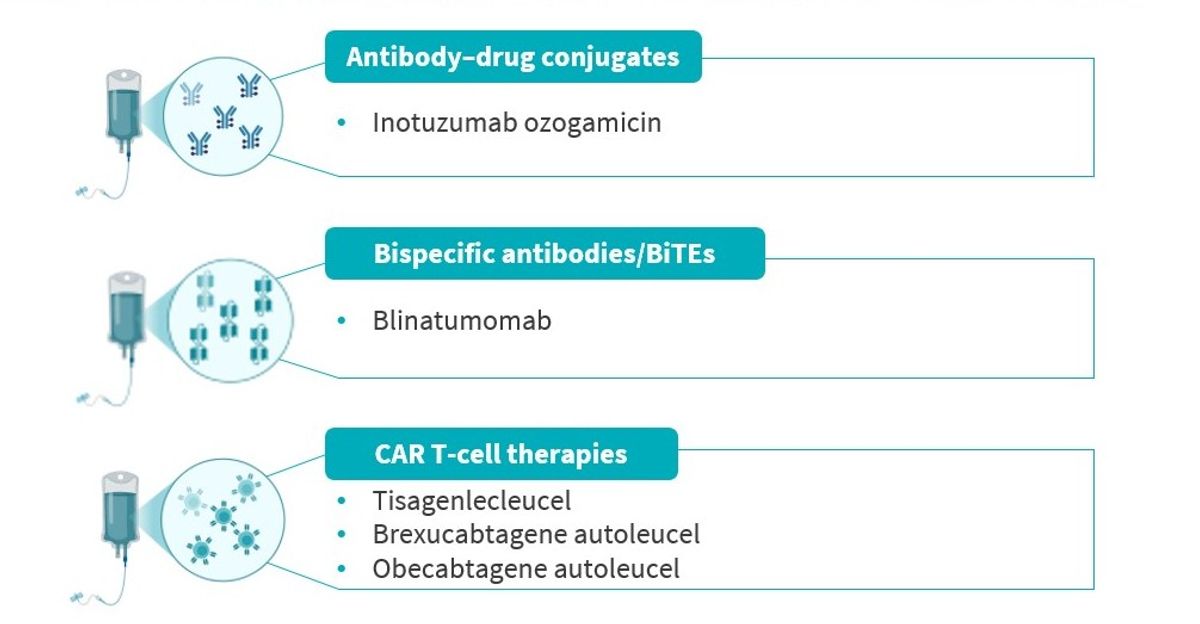
ALL, acute lymphoblastic leukemia; BiTE, bispecific T-cell engager; CAR, chimeric antigen receptor; EU, Europe; US, United States.
Aureli A, et al. Cancers (Basel). 2023;15(13):3346.
- Inotuzumab works by attaching to a protein called CD22 on leukemia cells. Once attached, it delivers chemotherapy directly into the cancer cell, sparing most healthy cells. It is approved for adults and children (over 1 year old) if their leukemia cells carry CD22, and it is being studied in earlier treatment settings and in combination with other therapies.
- Blinatumomab acts as a bridge between leukemia cells (with CD19) and the patient’s immune cells (T cells, with CD3), which guides the immune system to attack leukemia cells. It is approved for children (over 1 month old) and adults, in both relapse settings, and when small amounts of disease remain (MRD+).
- CAR T-cell therapies involve collecting a patient’s (or donor’s) T cells, changing them in a lab to recognize leukemia, and giving them back to the patient. This can cause very strong reactions when they start working, and patients usually need hospital care during the first days or weeks after treatment. CAR T-cell therapies are approved for certain patients with relapsed or refractory B-cell ALL.
- Immunotherapy offers new, highly targeted ways to treat B-cell ALL.
- So far, immunotherapies are only approved for B-cell ALL.T-cell ALL (T-ALL) is harder to treat with immunotherapy, because the cancer cells look too similar to normal T cells.
- Research is ongoing into new drugs and CAR T-cell therapies that target T-cell leukemia: using immunotherapy earlier in treatment, not just after relapse; developing faster, more accessible CAR T-cell therapies; and exploring ‘off-the-shelf’ CAR T cells made from donors, so patients don’t always have to wait for their own cells to be engineered.
- Side effects are different from (but can be as serious as) chemotherapy and require careful monitoring. Research is moving fast to make these treatments safer, faster, and available for more patients













































